Aseptic Sampling Hub
The increasing emphasis on closing process steps in bio-manufacturing is driven by the widespread adoption of single-use technologies, which address challenges such as open and manual operations that must be conducted in aseptic conditions. These innovations help minimize the risk of microbial contamination in aseptic environments, including those related to operator activities, ensuring product protection. Aligned with regulatory recommendations, BECARV’s Aseptic Sampling Hub is designed as a state-of-the-art, automated, and closed system to tackle these contamination risks, effectively closing the first step in the cell-based manufacturing process.
Additionally, streamlining manufacturing processes offers significant economic and operational benefits, such as the potential for cleanroom decommissioning, simplifying workflows, and improving reproducibility and safety, all while adhering to regulatory standards and ensuring the production of sterile products.
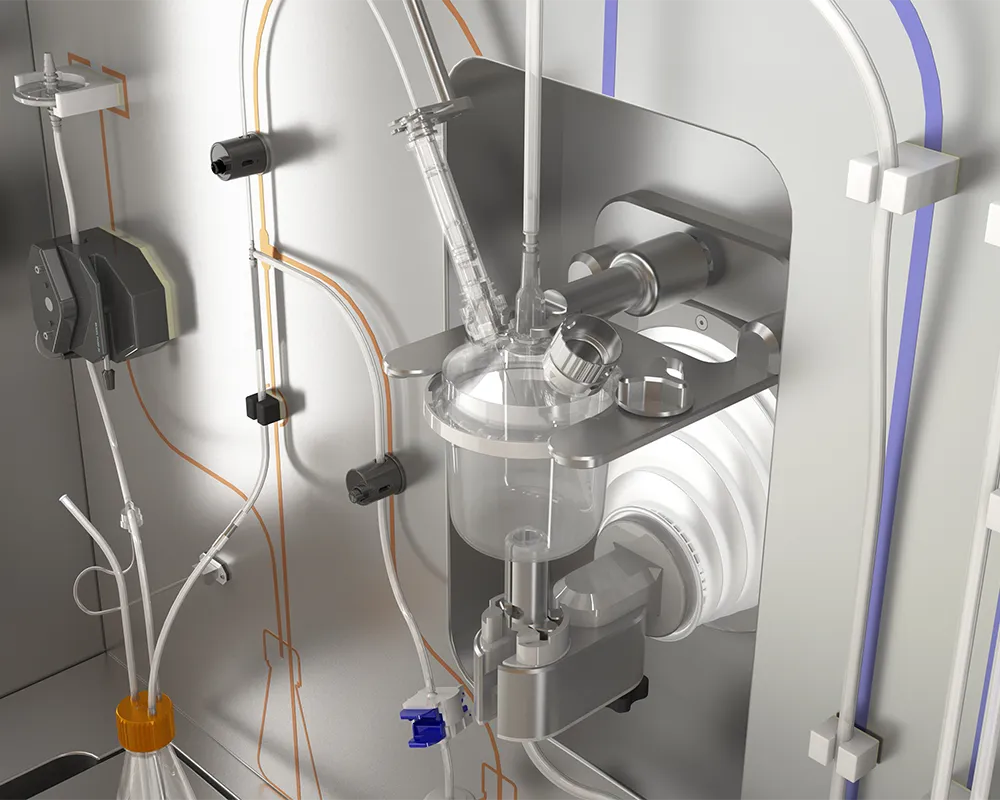
Aseptic Sampling Hub for Cell Line Inoculation
The Aseptic Sampling Hub is intended to enhance the quality and reliability of cell culture-based manufacturing processes by closing cell inoculation. This single-use system is equipped for self-disinfection, operates automatically, and includes sensors for data capture.
This groundbreaking device isolates the operator from the cell sample, thereby reducing contamination risks and improving the quality of Upstream Process (USP) manufacturing operations and product safety.
Key Features
- Single-use device
- Automated transfer of cells from vial to culture container
- Enhanced safety for both product and operators

Examples of Application
- Cell line inoculation
- Viral seed inoculation
- Sterile transfer of additives for media preparation
- Cell banking

Why choose the Aseptic Sampling Hub?
Self-Disinfecting Equipment, Single-Use kit
Equipped with automatic self-disinfection before cell handling, it ensures the equipment is sterile and contamination-free, significantly reducing contamination risks during cell line inoculation. The Aseptic Sampling Hub utilizes single-use, gamma-irradiated kits.
Automated, Integrated Operation
Fully automated, the Aseptic Sampling Hub integrates smoothly into your existing workflows. It captures and records critical process data in real time, ensuring accurate monitoring and traceability of operations, while complying with regulatory requirements and quality assurance standards.
Lower Room Grade for Operation
The closed environment for vial opening and cell transfer enables aseptic operations in a lower cleanroom classification—typically from Grade B (ISO5) to Grade C or D (ISO8). It eliminates the need for a dedicated cell line inoculation room and reduces operational costs, environmental monitoring and control requirements, access and gowning requirements, and associated training costs.
Higher Safety and Reliability
By preventing direct operator contact with cell samples, the Aseptic Sampling Hub enhances process safety and reliability. Its automated operation and real-time data capture increase reproducibility, ensuring greater consistency and control throughout the aseptic process.
FAQ
Why integrate this equipment into your existing bio-manufacturing process?
With the bio-pharmaceutical industry and regulators increasingly focused on controlling contamination risks in manufacturing, BECARV’s Aseptic Sampling Hub provides a reliable and cost-effective solution to close the final open step in cell culture-based bio-production, significantly reducing the risk of product contamination.
What is the total duration of the decontamination sequence using the Aseptic Sampling Hub, including decontamination, rinsing, drying, and cell transfer into the culture container?
The entire sequence is completed in a maximum of ten minutes. The duration can be further reduced when using hydrogen peroxide (H2O2) as the disinfecting agent, as the rinsing step can be skipped.
Does the Aseptic Sampling Hub accommodate different types of cryopreservation containers, such as glass and plastic cryovials or screwed cryotubes?
Yes, the Aseptic Sampling Hub supports all common types of cryovials, including glass and plastic cryotubes (1-5 ml, 12 mm diameter) and septum vials such as AT® vials (6 ml). It also accommodates screwed vials, as the device features a mobile chamber that unscrews the vial cap for content aspiration.
What are the components and parts of the Aseptic Sampling Hub?
The Aseptic Sampling Hub is a stand-alone unit featuring a stainless-steel cabinet mounted on wheels, equipped with pumps, sensors, and a robotic arm. The single-use disposable includes a semi-flexible/rigid disinfection chamber, tubing lines, and aseptic connectors that are plugged into the cabinet. The disposables provided by BECARV are pre-sterilized through gamma-irradiation, double-wrapped, and ready for use. Cell culture bottles and disinfecting/rinsing agents are not included, as they are process-specific and can be selected by the customer. The waste bottle, pre-assembled in the disposable system, has a five-liter capacity and connects to the system via aseptic connectors or tube welding.